PH description and scale
pH Defined
pH measures the acidity or basicity of a solution. It quantifies the concentration of hydrogen ions in a liquid, with values below 7 indicating acidity, 7 being neutral (like pure water), and values above 7 indicating basicity. The pH scale measures whether there is more hydronium or hydroxide in a solution. In other words, it tells us how basic or acidic the solution is. A lower pH means something is more acidic, also known as a stronger acid. A higher pH means it is more alkaline or a stronger base.
Chemistry classes will often use a litmus test to identify acids from bases. A blue litmus paper turns red in acids while a red litmus paper turns blue in basic solutions. Other pH indicator papers are available that will actually identify the rough pH of some acid or base, also using color-change chemicals.
At 25 °C (77°F), solutions with a pH less than 7 are acidic, and solutions with a pH greater than 7 are basic. Solutions with a pH of 7 at 25 °C are neutral (i.e. have the same concentration of H+ ions as OH− ions, i.e. the same as pure water). The neutral value of the pH depends on the temperature and is lower than 7 if the temperature increases above 25 °C. The pH range is commonly given as zero to 14, but a pH value can be less than 0 for very concentrated strong acids or greater than 14 for very concentrated strong bases.[2]
Why pH Measurements Are Important
Chemicals reactions in water are affected by the acidity or alkalinity of the solution. This is important not only in the chemistry lab, but in industry, cooking, and medicine. pH is carefully regulated in human cells and blood. The normal pH range for blood is between 7.35 and 7.45. Variation by even a tenth of a pH unit may be fatal. Soil pH is important for crop germination and growth. Acid rain caused by natural and man-made pollutants changes the acidity of soil and water, greatly affecting living organisms and other processes. In cooking, pH changes are used in baking and brewing. Since many reactions in everyday life are affected by pH, it’s useful to know how to calculate and measure it.
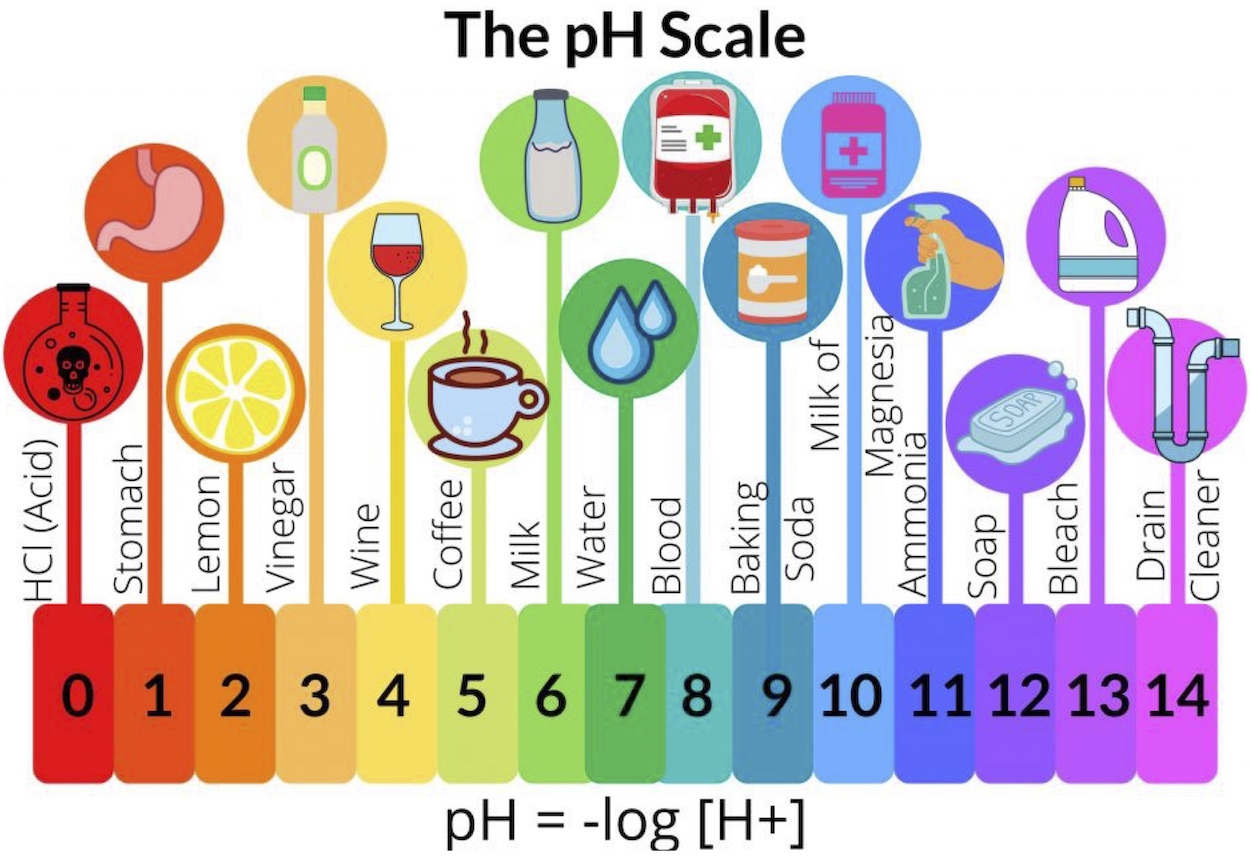
Most images show the pH scale going from zero to 14. This scale is logarithmic, so there is a 10-fold difference in strength between each number.
Pure water is neutral, neither an acid nor base. As such, it sits smack in the middle of the pH scale at 7. But mix an acid with water and the water molecules will act as bases. They’ll snag hydrogen protons from the acid. The altered water molecules are now called hydronium (Hy-DROHN-ee-um). Mix water with a base and that water will play the part of the acid. Now the water molecules give up their own protons to
the base and become what are known as hydroxide (Hy-DROX-ide) molecules.
Fun Facts About the pH Scale
- Acids often have a sour taste to them, like lemons
- Bases tend to taste bitter
- Søren Peder Lauritz Sørensen devised the pH formula and pH scale in 1909. Sørensen was a Danish chemist working at the Carlsberg Laboratory.
- Many cleaners are very basic (pH > 10). Some examples include drain cleaner, bleach and ammonia solution. Always be careful working with very basic or very acidic substances.
- Due to the increase in carbon dioxide in the air, our oceans are currently decreasing in pH which means they are getting more acidic. The change in pH has cascading effects on the creatures in the ocean.
